Carbon Capture, Utilization and Storage (CCUS) is considered to be the most effective method of mitigating the greenhouse effect. Geological sequestration of CO2 is a core component of CCUS, where the advantages of mineral capture are its strong thermodynamic reactivity, low leakage potential, and the potential to permanently sequester CO2. Dawsonite is widely found in CO2-filled deep saline or sedimentary reservoirs, and is considered to be one of the important carbon capture minerals in CCUS projects. Numerous numerical simulations have shown that if sufficient amounts of sodium-rich feldspar and CO2 are present in sandstone formations, large amounts of dawsonite can be easily formed to achieve permanent CO2 sequestration. In addition, dawsonite is also commonly used as a flame retardant, preservative, in the aluminum industry, and as a precursor for ceramics. Therefore, exploring the optimal conditions for the rapid and large-scale synthesis of dawsonite using CO2 is of great significance for the geological sequestration and industrial utilization of CO2.
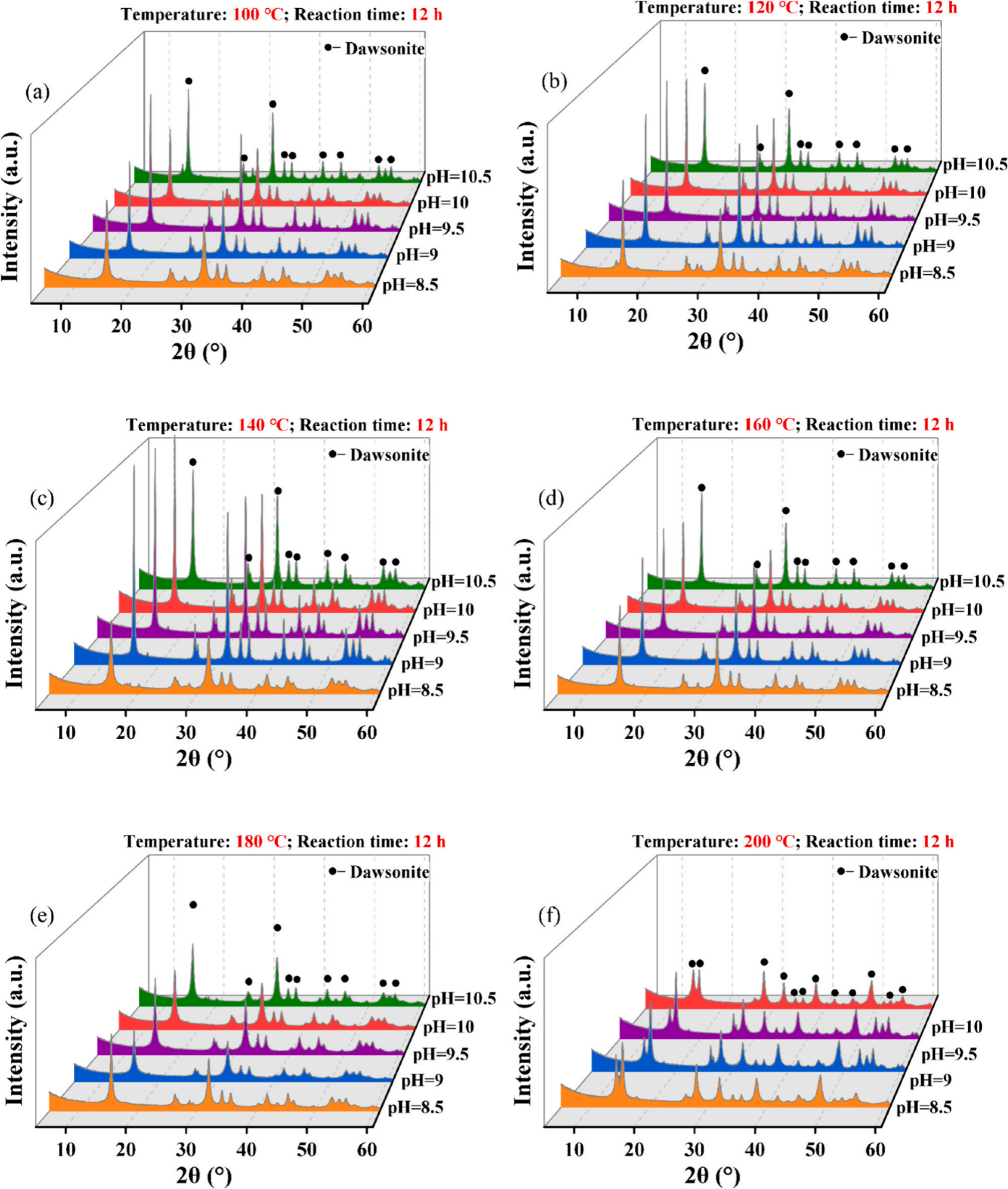
Fig 1. XRD patterns of dawsonite under the same temperature and different pH conditions.
Currently, there are many methods for synthesizing dawsonite, but they are not specifically aimed at CO2 sequestration, and most of the synthetic methods rely on chemical reagents containing carbonate or bicarbonate ions as precursors, with limited participation of CO2, and the best synthetic conditions are still controversial. Therefore, it is worth further study to explore how to quickly and massively synthesize dawsonite with the participation of CO2 to achieve CO2 mineralization sequestration. Dr. Yangchen Zhang from the School of Geoscience, under the guidance of Associate Prof. Qu Xiyu, based on physical simulation experiments, XRD, and SEM, used AlCl3·6H2O, NaOH, NaCl, and CO2 as the initial raw materials to synthesize dawsonite by hydrothermal method. The purity, morphology, size and quality of dawsonite synthesized under different conditions were compared, the optimal conditions for synthesizing dawsonite using CO2 were identified, the hydrothermal synthesis mechanism of dawsonite was discussed, and the CO2 sequestration potential of dawsonite was preliminarily evaluated. The results show that pure dawsonite can be synthesized at a pH value of 8.5–10.5, and a temperature of 100–180 ℃, with a reaction time of ≥6 h (Fig. 1); the temperature having a more pronounced effect on the purity and morphology of dawsonite; extending the reaction time has a minimal impact on the synthesis amount of dawsonite; when the temperature is 140 ℃ and the pH value is 9.5, the synthesis amount and CO2 mineralization rate of dawsonite reaches the maximum values. This indicates that these temperature and pH conditions may be the best experimental conditions for hydrothermal synthesis of dawsonite using CO2, and may also be the ideal condition for CO2 mineralization sequestration. At 50 ℃, the nucleation rate of dawsonite is much larger than the growth rate, leading to agglomeration; when the temperature reaches 100 ℃, both the nucleation and the growth rates increase, and the granular dawsonite crystals gradually grow into needle-like along a certain direction; at 120–160 ℃, the growth rate is larger than the nucleation rate, the homogeneity and dispersion of the crystals are enhanced, and the diameter increases; at 180 ℃, both the growth rate and nucleation rate decrease, the synthesis amount of dawsonite decreases significantly, and the crystal diameter decreases; when the temperature is 200 ℃, dawsonite begins to dissolve, and the pseudo-boehmite appears (Fig. 2).
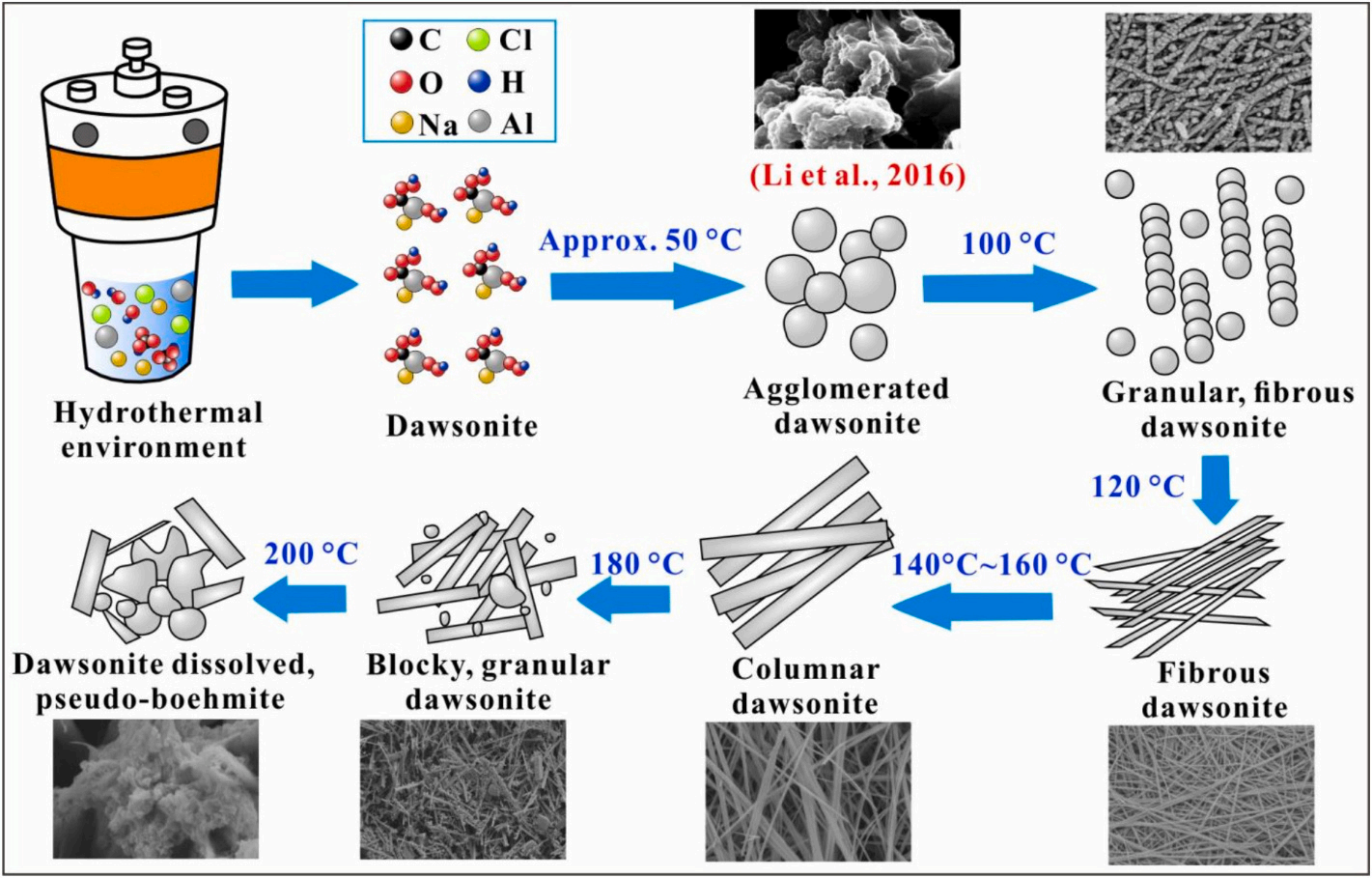
Fig 2.Hydrothermal synthesis mechanism of dawsonite.
Paper Information:
Yangchen Zhang, Xiyu Qu*, Yong Yuan, Yinguo Zhang, Qian Li. 2024. Mineral trapping of carbon dioxide: Rapid hydrothermal synthesis experiments and carbon sequestration potential of dawsonite. Science of The Total Environment, 946, 174220. https://doi.org/10.1016/j.scitotenv.2024.174220.

